Electrochemistry
Electrochemistry is a powerful tool that uses electrodes to interface between chemical reactions and electricity. And when molecules get close enough, they can exchange electrons with the electrode surface! A shocking number of molecules in nature are capable of interacting with electrodes, they give or receive electrons and we measure the current through the electrode that results to know just how much of that molecule is there.
|
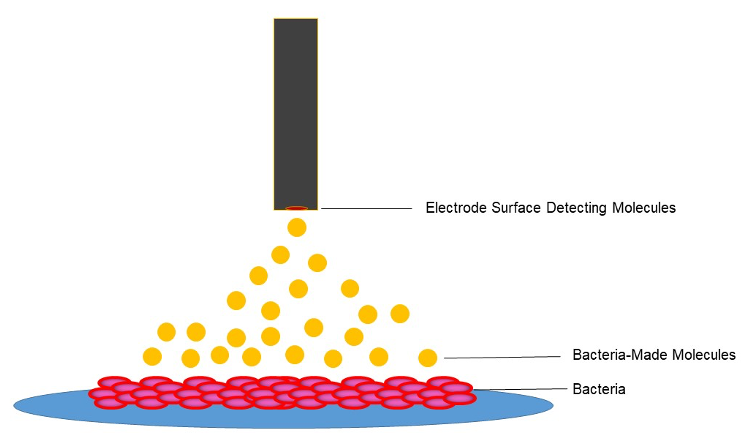
In nature, bacteria use molecules as a way to communicate with neighbors, kill enemy strangers, and protect themselves from harm. In our lab, we use electrochemistry to measure the presence and concentration of molecules and study how bacteria use them in different conditions. Since bacteria are incredibly small, we use micron sized electrodes to get up close and personal when doing these experiment!
|
|
We begin by manufacturing our own micron-sized electrodes using equipment built precisely for this purpose. These electrodes are crafted by hand and are specific for the molecule we are interested in studying. The figure on the right shows the tip of a microelectrode with a 25 µm wire visible in the center. |
On the bacterial side of things, cells are grown up depending on what questions we want to ask. Is one bacteria going to have a different concentration gradient of a molecule when it’s near a neighbor? How does spatial structure impact the concentration gradient of molecules? What happens to concentration gradients when we start to disturb bacteria in their environment?
|
To answer these questions, we use a potentiostat–this instrument controls the electrodes and the chemical reactions happening on the surface. Using a potentiostat, we are able to measure bulk concentrations of molecules, characterize media environments, and even observe interactions between bacteria and electrodes. Once positioned, the electrode can directly measure the local concentration and redox state of small molecules surrounding a bacterial population in real time. We do this by controlling the electrode potential using a computer.
|
If you can imagine, a sea of electrons resides on the surface of the electrode and by decreasing or increasing their energy (by changing the electrode potential), we can take or remove electrons from molecules. And, by changing the potential we can probe the concentration and redox state of multiple different electroactive molecules throughout an experiment.
The Scanning Electrochemical Microscope
|
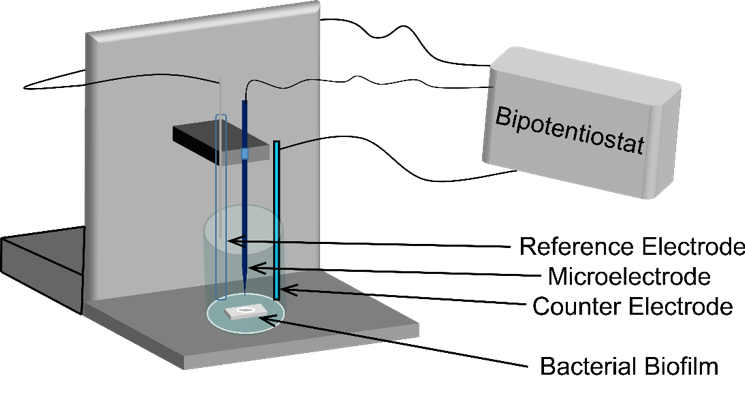
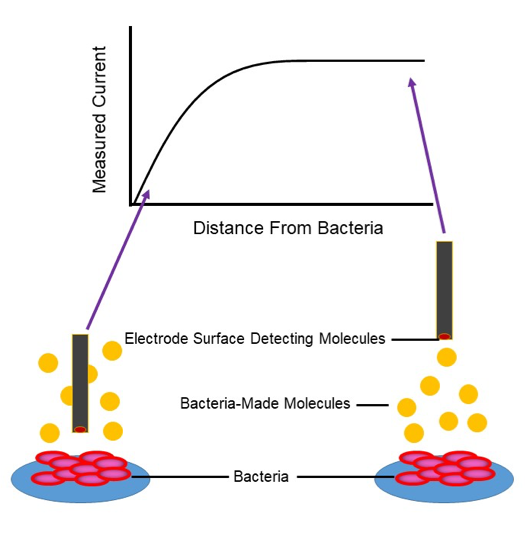
Bacteria are small, and transient chemical landscapes are often difficult to characterize. Therefore, we couple the power of a potentiostat with the preciseness of a movable stage that positions electrodes within micron-scale distances; this instrument is called a Scanning Electrochemical Microscope (SECM). The SECM sets the distance between bacteria and the microelectrode using a feedback approach curve; as the electrode gets close to the bacteria, molecules cannot diffuse so easily to the surface and we measure less current. By knowing something about where this drop-off in signal occurs, we can define how far away our electrode is and move it accordingly to the precise location we want.
|
Diagram of the Scanning Electrochemical Microscope.
|
|
Molecules easily diffuse to the surface when the electrode is far away (right), and molecules are blocked from the electrode surface near the bacteria resulting in decreasing current (left).
Scanning the tip in the x, y, or z direction creates a spatially resolved map of the molecule along a surface. Since this method provides quantitative information in real time, monitoring a biological system over time allows SECM to generate a spatio-temporal profile of a dynamic environment! We work in collaboration with Dr. Allen Bard’s group at UT Austin to characterize bacterial behaviors in complex, dynamic environments using SECM. We have employed SECM to spatially map hydrogen peroxide production in mixed species biofilms containing Streptococcus gordonii and Aggregatibacter actinomycetemocomitans., |
We have also demonstrated that Pseudomonas aeruginosa biofilms produce and maintain an “electrocline” (a gradient of redox potential) of pyocyanin, a quorum sensing signal and antimicrobial molecule, in its reduced form surrounding the biofilm. We continue to work together with the Bard group to use SECM to probe bacterial interactions among small populations of bacteria and individual cells.
Selected Work:
https://www.pnas.org/content/108/50/19996
https://www.pnas.org/content/108/7/2668.short
https://www.pnas.org/content/111/51/18255
Selected Work:
https://www.pnas.org/content/108/50/19996
https://www.pnas.org/content/108/7/2668.short
https://www.pnas.org/content/111/51/18255